CLOSING THE GAP: NEW MALARIA TREATMENT OFFERS HOPE FOR VULNERABLE NEWBORNS
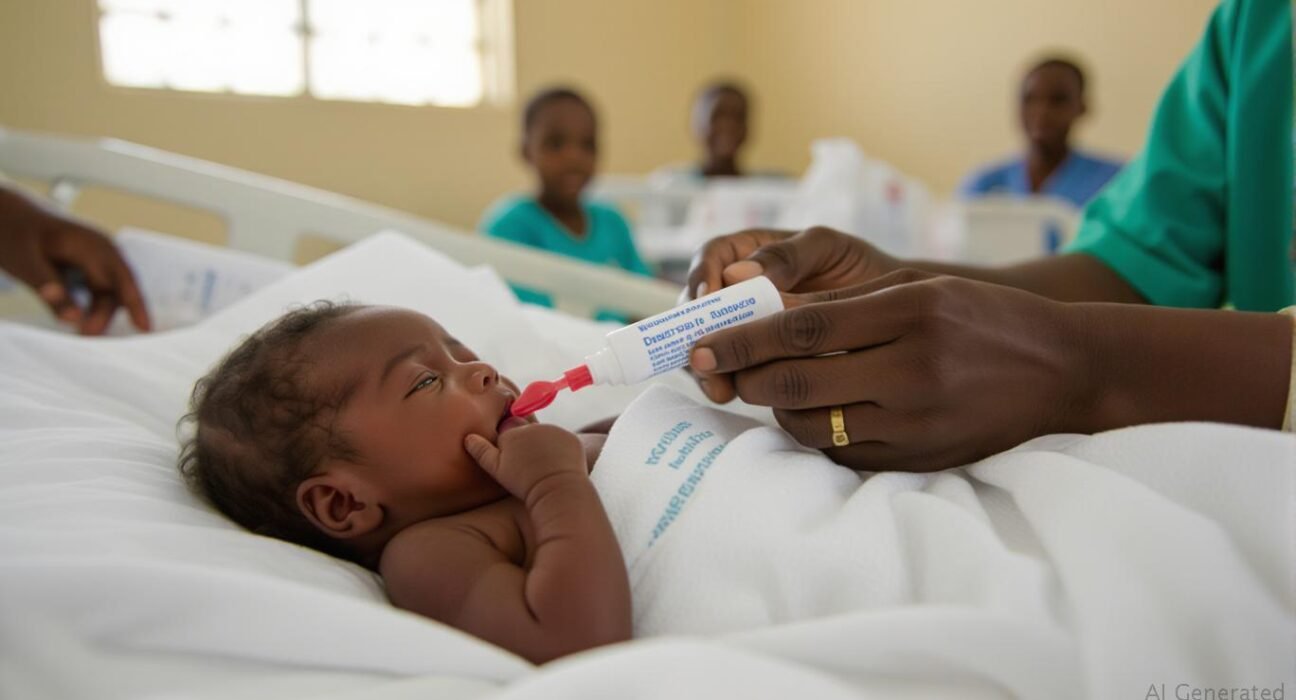
A newly approved malaria treatment tailored specifically for newborns and very young children has been hailed as a significant advance in the fight against one of Africa’s deadliest diseases.
Swiss regulators have given the green light to the drug, known as Coartem Baby (also marketed as Riamet Baby), which is expected to be rolled out in several African countries within weeks. The continent continues to bear the highest burden of malaria-related deaths, particularly among children under five.
Until now, there has been no malaria treatment officially approved for babies weighing under 4.5kg (approximately 10 pounds). Healthcare workers have often had to adapt medications designed for older children—an approach that carried risks due to infants’ developing liver function and distinct dosing needs.
A TREATMENT GAP EXPOSED
Medical experts have welcomed the approval as a long-overdue response to a “critical treatment gap” that has persisted for decades. Using formulations not suited to infants has led to dosing challenges and increased the risk of adverse effects.
In 2023, malaria claimed an estimated 597,000 lives globally—most of them in sub-Saharan Africa. According to the World Health Organization, around 75% of these deaths occurred in children under the age of five, underscoring the urgent need for infant-specific interventions.
GLOBAL COLLABORATION BEHIND THE BREAKTHROUGH
The new treatment was developed by Novartis in partnership with the Medicines for Malaria Venture (MMV), a Swiss-based nonprofit backed by international donors, including several governments and the Bill & Melinda Gates Foundation.
Clinical trials were conducted in eight African nations, which are expected to be among the first to receive the new formulation. The drug is a modified version of the widely used Coartem, adjusted in both dosage and delivery to meet the specific needs of newborns.
Novartis CEO Vas Narasimhan described the approval as a “vital milestone” in global health. “We’re proud to have developed a solution for even the smallest and most vulnerable patients,” he said, noting the company’s longstanding commitment to malaria innovation.
ACCESS AT THE HEART OF THE ROLLOUT
The treatment will be made available on a not-for-profit basis, a decision welcomed by global health advocates who have long called for greater equity in drug access. Experts say the model could help bridge disparities in care and improve outcomes for infants in some of the world’s most underserved communities.
Health officials and humanitarian agencies hope that the launch of Coartem Baby marks the beginning of a broader push to close remaining gaps in malaria care—and save the lives of children who are too often left without effective treatment.









